Department of Chemical Pathology

DEPARTMENT OF CHEMICAL PATHOLOGY
QUALITY OF CARE POLICY AND OPERATIONAL GUIDELINES
NHA/CD/QOCP/CHP/001
ISSUE DATE: 19/11/2021
VERSION 1
Dr. Nabila Abubakar
HEAD OF DEPARTMENT


1.0 INTRODUCTION
The Department of Chemical Pathology, National Hospital Abuja was established in 1999 as one of the 4 units of the then Pathology Department founding departments in NHA. It was subsequently expanded and metamorphosed into a separate department in 2002, providing quality Clinical Chemistry services to clients all over the nation. It is one of four separate but related departments (Chemical pathology, Haematology, Histopathology and Medical Microbiology) that constitute the entity – Pathology/Laboratory Medicine. The Department is fully accredited for residency training by the West African College of Physicians (WACP) and National Postgraduate Medical College of Nigeria (NPMCN). The department is also involved in training of house officers, intern medical laboratory scientists, corps members and students on industrial attachment.
1.1 Vision Statement
To be the foremost provider of quality laboratory services in Chemical Pathology including clinical chemistry, endocrinology and metabolic medicine in the country, reducing the need for medical tourism.
1.2 Mission Statement
Our goal is to provide quality, prompt, courteous, comprehensive and effective services leveraging on the use of state-of-the-art technology in a clean, conducive and patient-friendly environment.
2.0 QUALITY ASSURANCE POLICY
The Department of Chemical Pathology, National Hospital, Abuja in line with the mission and vision of the hospital is committed to the delivery of effective and efficient high quality chemical pathology laboratory services to our clients without compromise. We are committed to total customer satisfaction and compliance with ISO 15189 and other regulatory bodies to achieving our objectives. Our core values are Quality, Empathy and Dedication.
3.0 STRENGTH OF THE DEPARTMENT
The Department of Chemical Pathology has state-of-the-art automated clinical chemistry and immunoassay analyzers that produce a vast array of basic clinical chemistry and metabolic panel tests, as well as tumour markers and hormones. The department also has competent personnel who are committed to efficient service delivery and generation of quality laboratory reports to facilitate patient/client care.
In line with the vision of the hospital, the department is poised to ensure quality health service delivery to Nigerians and clients from other parts of the world. The department of Chemical Pathology consists of health professionals in Laboratory Medicine, Medical Laboratory Science and other related science specialties. It is headed by a Consultant Chemical Pathologist, who is a medical doctor with fellowship qualifications from either the WACP, NPMCN or its equivalent.
The head of department is supported by other Consultant Chemical Pathologists, Resident Doctors in Chemical Pathology, Medical Laboratory Scientists, Medical Laboratory technologists, Medical Laboratory Assistants, Scientific officers and other administrative staff including the departmental secretary who coordinates the departmental secretariat. The departmental management committee chaired by the HOD is the organizational think-tank of the department, comprising of staff on GL 14 and above and other designated senior staff.
Specialized services offered in the Department Of Chemical Pathology include:
- ROUTINE CLINICAL CHEMISTRY
- Renal function tests: Electrolytes, urea, creatinine, uric acid, 24 hour creatinine clearance
- Tests of Bone and mineral metabolism: Plasma Calcium, Phosphate, Magnesium assays
- Liver function tests/Enzymology: Total and Conjugated bilirubin, Liver enzymes: ALT, AST, ALP, Amylase, Total protein, Albumin
- Glucose metabolism and glycaemic control: Fasting and Random plasma Glucose assays, Oral glucose tolerance tests, HbA1c assays
- CSF biochemistry: CSF glucose, CSF Protein
- Lipid profile: Total cholesterol, HDL-cholesterol, LDL-cholesterol and Triglyceride
- Infectious/inflammatory panel: C-reactive protein (CRP), high sensitivity CRP
- IMMUNOASSAY-BASED TESTS
- Tumour markers: Total and Free PSA, AFP, CEA, Carbohydrate antigens CA 125, CA 15-3, CA 19-9
- Fertility profile: FSH, LH, Oestradiol, Progesterone, Testosterone, Prolactin, DHEAS
- Anti-Mullerian hormone (AMH)
- Thyroid function test: TSH, Free T3, Free T4, Total T3, Total T4
- HBsAg quantitation
- Cortisol
- Ferritin
- DYNAMIC FUNCTION TESTS
- Water deprivation test
- Dexamethasone suppression test
- Synacthen test
- SEPARATION TECHNIQUES
- Haemoglobin quantitation by HPLC
- ALLERGY TESTING
- Plasma IgE Allergy test: Adult, Respiratory, Food and Paediatric allergy panels
- POINT-OF-CARE TESTING
- Cardiac biomarkers: Cardiac Troponin T, NT Pro-BNP, D-dimer
- Infectious panel: Procalcitonin, Beta 2 microglobulin
- TOXICOLOGY SERVICES
- Urine Toxicology screening
- CLINICAL SERVICES
- Clinical Consultation on Endocrine and Metabolic disorders and In-born Errors of metabolism
- Metabolic and Nutrition clinic
- TRAINING AND RESEARCH
- Residency training for Primary residents in Chemical Pathology
- Training of other residents in other Pathology sub-specialties
- Training of residents in Family Medicine and Internal Medicine
- Training of House officers and Intern Medical Laboratory Scientists
- Training of students on Industrial attachment and Youth Corps members
- Cordination of research in Chemical Pathology and Metabolic Medicine
- EVALUATION OF POINT-OF-CARE-TEST DEVICES
- Evaluation of hospital-based point-of-care-testing devices
- Evaluation of point-of-care devices of clients
4.0 OPERATIONAL GUIDELINES
- Ensure that all departmental services/tests are carried out with the highest sense of commitment to appropriate quality and efficiency.
- Operate in conformance with the requirements of the quality management system and procedures
- Free access of all staff to all departmental policy documents and standard operating procedures
- Ensuring patient confidentiality and client satisfaction
- Prompt service delivery and best practice procedure
- Multi-level quality assurance process
- Participation in proficiency testing and external quality assurance
- Ensure proper record keeping of laboratory results, ambient temperature checks, laboratory supplies etc
- Ensure good inter-personal and intra/inter-professional relationships to ensure optimal outcomes
- All tests results, having undergone required quality checks are reported in authorized format using the Laboratory Information System (LIS), by the Consultant Chemical Pathologist or his designee.
- To ensure proper inventory and stock management via electronic/manual data capturing to promote timely procurement of reagents.
5.0 WEEKLY ACTIVITIES AND SCHEDULES
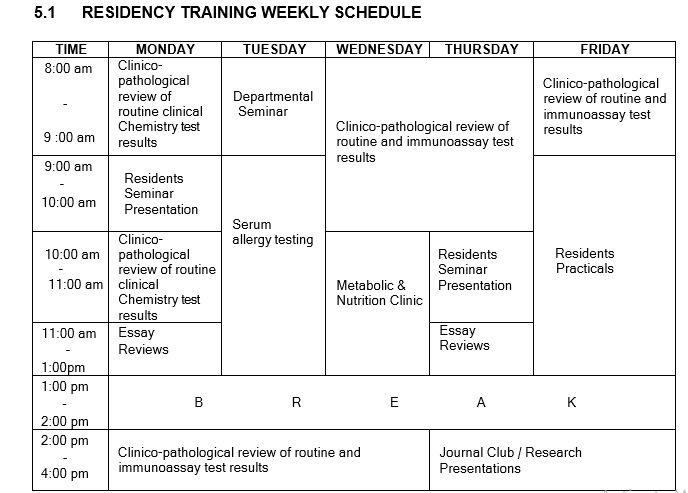
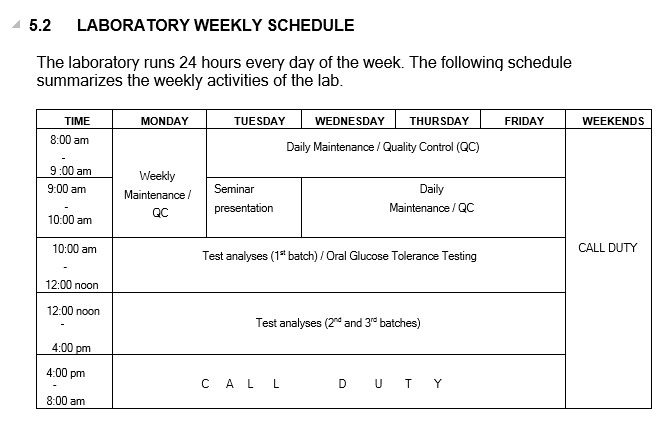
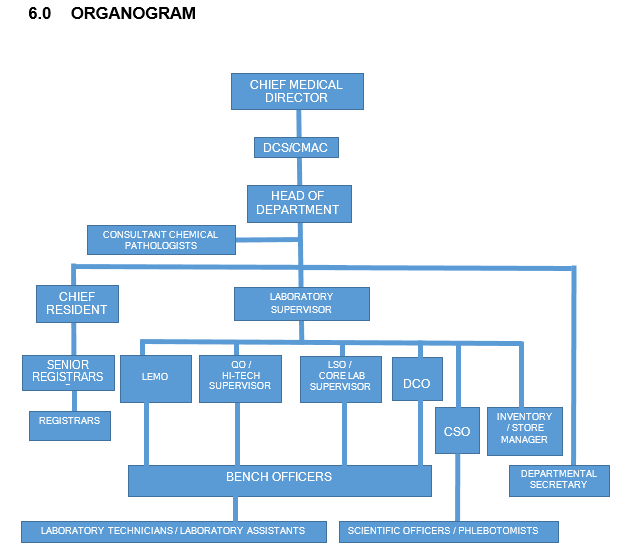
KEY: DCS – Director Clinical services; CMAC – Chairman Medical Advisory Committee; LEMO – Laboratory Equipment Maintenance Officer; QO – Quality Officer; LSO – Laboratory Safety Officer; DCO – Document Control Officer; CSO – Customer Service Officer.
7.0 HEALTH AND SAFETY POLICY
The Department of Chemical Pathology is committed to supporting the implementation of the National policy on injection safety and healthcare waste management with the following but not restricted to the following:
- To provide guidelines for the injection and other healthcare waste management practices to all laboratory
- To establish and ensure that proper injection and other healthcare waste management practices are observed at all sections of the laboratory and in the
- To ensure proper waste segregation and
Purpose:
This Safety Policy is to guide all staff in pursuing their responsibility to safeguard the health and well-being of everyone, staff, client and environment.
Responsibility:
- All laboratory staff
- Laboratory Safety officer
- Laboratory Management Committee
- Environmental management unit of the hospital
Key Elements:
- Establish and maintain adequate procedures, work practices and maintenance of buildings and equipment to ensure a safe working environment which covers many safety issues as follows:
- General safety
- Infection safety
- Chemical safety
- Specimen hazard control
- Hazard control measures
- Preventive measures
- Waste segregation
- Orient and train all staff in safe work practices and procedures in compliance with established rules and regulations and safety manual.
- The departmental management committee provides support and demonstrates action specific to the following:
- Resources: Advocate for sufficient resources to provide and maintain safe and healthy working conditions
- Training: Coordinate training in health and safety as well as first aid/fire drills
- Data collection: collecting and analyzing data on accidents, sickness and injury
- Develop safety awareness campaigns
8.0 MINIMUM STANDARDS POLICY
The department would maintain a framework through which all staff are accountable for continuously improving the quality of our services and safeguarding high standards of care, by creating an environment in which excellence in pathology practice will flourish.
9.0 LIST OF STAFF AND QUALIFICATIONS
The current members of staff of the Department of Chemical Pathology and their respective qualifications are listed as follows:
|
S/N |
CURRENT STAFF |
QUALIFICATION |
DESIGNATION |
|
|
CONSULTANTS (3) |
||||
|
1 |
DR J.A.F. MOMOH |
MB;BS, MSc, FWACP (Lab. Med) |
CHIEF CONSULTANT |
|
|
2 |
DR N.D ABUBAKAR |
MB;BS, FMCPath |
CONSULTANT |
|
|
3 |
DR. A.O. LAWAL |
MB;BS, MPH, MWACP (Lab Med), FMCPath |
CONSULTANT |
|
|
RESIDENTS DOCTORS (3) |
||||
|
1 |
DR.F. U. AGADA |
MB;BS |
SENIOR REGISTRAR I |
|
|
2 |
DR. J.A. MOHAMMED |
MB;BS |
SENIOR REGISTRAR I |
|
|
3 |
DR. D.E. UYA |
BMLS, MBBS |
SENIOR REGISTRAR I |
|
|
MEDICAL LAB SCIENTISTS (12) |
||||
|
1 |
DR ANTHONY ILEGOGIE |
AMLSCN, MSc, FMLSCN, PhD |
DDMLS |
|
|
2 |
DR AKHAUMERE EMMANUEL |
AMLSCN, MSc, PhD |
ADMLS |
|
|
3 |
AKPORHUARHO CATHERINE |
AMLSCN, PGD |
ADMLS |
|
|
4 |
OJO-DANIEL ADESUA |
AMLSCN, MSc |
ADMLS |
|
|
5 |
OBAJI-OGAR LARA |
BSc, MSc, AMLSCN |
ADMLS |
|
|
6 |
IFEANYI MORAH |
BMLS |
SMLS |
|
|
7 |
LILIAN JOSEPH |
BMLS |
SMLS |
|
|
8 |
EUNICE DABI |
BMLS |
SMLS |
|
|
9 |
CHARITY AGARANYA |
BMLS |
SMLS |
|
|
10 |
SONIBARE OLAWALE OLADIMEJI |
BMLS |
SMLS |
|
|
11 |
DR ANYA DORATHY |
BMLS, PhD |
MLS I |
|
|
12 |
CHUKWUEKEZIE AMARA |
BMLS |
MLS I |
|
|
SCEINTIFIC OFFICERS (3) |
||||
|
1 |
OLASIKU TAIYE |
HND, SLT |
CSO |
|
|
2 |
ALIYU IBRAHIM |
BSc |
CSO |
|
|
3 |
EFFIONG UBOKOBONG O. |
BSc |
SO |
|
|
MEDICAL LABORATORY TECHNICIANS (6) |
||||
|
1 |
ANGELA SAMUEL |
MLT |
MLT |
|
|
2 |
BERNARD AIKOYE |
MLT |
MLT |
|
|
3 |
RIFKATU ABASIA |
MLT |
MLT |
|
|
4 |
PLAGNAN MWANTU |
MLT |
MLT |
|
|
5 |
ESTHER DOYINSOLA |
MLA |
MLT |
|
|
6 |
YAKUBU IBRAHIM |
MLT |
MLT |
|
|
MEDICAL LABORATORY ASSISTANTS (6) |
||||
|
1 |
ODEKINA CHRISTOPHER |
MLA |
LAB. ASSISTANT |
|
|
2 |
UMAR MOHAMMED ADAMU |
MLA |
LAB. ASSISTANT |
|
|
3 |
YAKUBU ADAMU |
MLA |
LAB. ASSISTANT |
|
|
4 |
YAHAYA HUSSAINI |
MLA |
LAB. ASSISTANT |
|
|
5 |
MANYA MAVEL |
MLA |
LAB. ASSISTANT |
|
|
6 |
NALADO AHMED |
ND (SLT) |
LAB. ASSISTANT |
|
|
HOSPITAL ASSISTANT (1) |
||||
|
1 |
MOHAMMED MARYAM |
SSCE, BSc |
HOSPITAL ASSISTANT |
|
|
DEPARTMENTAL SECRETARY (1) |
||||
|
1 |
COMFORT H. EMUEKPERE |
HND |
CONFIDENTIAL SECRETARY |
|
Contact email : hod.chempath@nationalhospital.gov.ng